TISSUE ENGINEERING
from petri dish to patient
Tissue engineering (TE) is a multidisciplinary, revolutionary field of regenerative medicine which seeks to restore, sustain, or replace damaged tissues or organs made of constructs of cells, scaffolds, and other biomolecules.
WHAT IS TISSUE ENGINEERING?
Tissue engineering all began as a possible solution to a widespread health problem in the United States, tissue and organ failure. Over half of the total annual expenditure in health care in the US is due to tissue and organ problems. Before tissue engineering, the only possible treatmeant options were transplantation, surgical repair, and possibly drug therapy. However, these solution only worked on minor damage and couldn't cause long-term recovery, thus sparking a need for another treatment option for those who experienced major damage to their tissues or organs.
Surgeons faced a dilemma that brought about a need for tissue engineering, the need to replace non-functioning organs or body structures without causing additional damage due to tissue loss. In addition, some vital organs could not be removed without a substitute apparatus being introduced to mimic the organ’s function in the body. Because of this, doctors looked to reconstruct anatomical structures using the patient’s own cells as the raw material.
However, the idea of tissue engineering was first vocalized by Y.C. Fung, a pioneer in the field of bioengineering, who submitted a proposal in 1985 for a research facility to be built called the "Center for the Engineering of Living Tissues." He argued that while there had been extensive research on cells and organs as seperate entities, there had been few efforts focused on understanding the gap between the two. His proposal was later rejected, but it brought to the forefront the idea of using an engineering approach towards the biological organization between cells and organs.
Just two years later, a panel meeting was held by the National Science Foundation (NSF) to discuss the use of engineering in accordance with cells and organs. Fung was also present at this meeting, and volunteered the term "tissue engineering" to describe the concept of growing up a cell into an organ. From then on, many scientists prepared definitions for tissue engineering, each building on the previous drafts. And as the field of tissue engineering grew, the ideas put forth by Fung started to change from mere thoughts into actions, and solutions were being delivered from petri dish to patient.



HOW IT ALL STARTED
HOW TISSUE ENGINEERING WORKS
AUTOLOGOUS CELLS
are extracted from the patient and grown in vitro (in a laboratory), in order to increase the number of cells. Using autologous cells is preferred over the other two cell source types because there is no risk for immunological rejection in the host. However, only small samples may be taken from the patient since tissue engineers must take cells from the organ or tissue that is damaged and under repair.

The process of autologous stem cell therapy (I2.6)
However in tissue engineering, these “scaffolds” can be replicated with materials from external or synthetic sources, like plastics and proteins. Also, existing scaffolds can also be taken from the tissues of a donor organ. The cells are removed from the collagen scaffold, which allows for the growth of new tissue growth on the scaffold using the patient’s own cells. This eliminates the issue of the rejection of foreign cells by the patient’s immune system since his or her own cells would be integrated within the tissue.

WHAT ARE SCAFFOLDS?
After choosing the cells, it is time to choose a scaffold. Scaffolds are essential components in the creation of tissue engineered products. A product without a scaffold is known simply as "cell therapy."
Though the cell is the fundamental unit of life, tissues wouldn’t function without the structure surrounding and secreted by the cells called the extracellular matrix. The extracellular matrix around cells support the cells and allows for intercellular signaling and communication. While all extracellular matrices are made of the essential components of water, polysaccharides, and proteins, specific types of cells or tissue have their own composition specific to their microenvironment and function.
From a young age, we learn that cells are the building blocks of life. But what does that mean? The human body has trillions of cells, many of them differentiated into skin cells, liver cells, heart cells, lung cells, and many, many more types of cells.
However, not all cells have developed into special cells with unique functions; stem cells are undifferentiated, which means that they can become any type of cell in the body. The stem cells have the ability to differentiate under certain conditions, whether artificially induced or naturally produced, and become the specialized cells in our eyes, nose or cheek.

The cloning of adult stem cells. I2.1
AN INTRODUCTION TO CELLS
AN OVERVIEW OF HOW IT WORKS:
In tissue engineering, a scaffold made of natural or biosynthetic materials is used as a foundation for cells to grow on. The cells are seeded into the scaffold along with other biomolecules and growth factors to initiate tissue growth. Once the final product is formed, it can be fully integrated into living systems. The end product of tissue engineering can range from bioengineered organs to synthetic meat, which shows the varied applications of tissue engineering.

THE DIFFERENT TYPES OF CELLS USED IN TISSUE ENGINEERING:
1. Autologous cells: from the patient
2. Allogeneic cells: from another human
3. Xenogeneic cells: from an different species
XENOGENEIC CELLS
are cells acquired from a different species. The use of animal tissues requires immunosuppression because our bodies recognize their cells as foreign and harmful to our native cells. Xenogeneic cells are generally used in skin (epidermal) transplants. Animals, such as the pig, are genetically modified so that their cells mimic human cells by negating pig-specific genes and expressing human ones.

Xenogeneic transplants often use pigs as donors (I2.8)
ALLOGENEIC CELLS
are taken from another human source and are often used for skin transplants and regrowth in tissue engineering since they can release potent growth factors to promote regeneration. Therapies using allogeneic cells require immunosuppression treatments to inhibit the immune system from attacking the transplant as a harmful foreign body.

Allogeneic stem cell transplantation from the perspective of cancer treatment (I2.7)
STEM CELLS CAN ALSO BE CLASSIFIED BY EXTENT OF DIFFERENTIATION:
1. Pluripotent embryonic stem cells (ESCs)
2. Multipotent adult stem cells (ASCs)
3. Induced pluripotent stem cells (iPSCs)
PLURIPOTENT EMBRYONIC STEM CELLS

An image of the extraction of ESCs (I2.9)
are completely undifferentiated stem cells and can specialize into any type of cell. This allows for tissue engineers to craft any human tissue from these cells. These stem cells are taken from embryos (Embryonic Stem Cells, or ESCs), which has caused public concern about the ethics of this method. However, human ESCs (hESCs) are taken from embryos fertilized and grown in vitro with full consent from the egg donor. During growth, a feeder layer of mouse cells may be added to the petri dish prior to the addition of the ESCs to allow for adhesion to the surface and to provide nutrients into the culture.
MULTIPOTENT ADULT STEM CELLS
are partially differentiated somatic stem cells that can only specialize into certain types of cells related to a specific organ. For example, if a pluripotent stem cell develops into a blood stem cell (a hematopoietic stem cell), it becomes a multipotent stem cell. This multipotent stem cell can only become types of cells limited to the blood, like red and white blood cells.
Multipotent stem cells can come from adults, and can thus be classified as Adult Stem Cells or ASCs. ASCs occur in blood, mesenchymal, muscle, epidermal, and endothelial tissues, among others.
Another type of ASC is the mesenchymal stem cell which forms bone, fat cells, and cartilage. The structural progeny of mesenchymal stem cells make them extremely useful in tissue engineering applications like in cartilage engineering.

Graphic showing the differentiation of mesenchymal stem cells (I2.10)
INDUCED PLURIPOTENT STEM CELLS
A video detailing the process of reprogramming adult somatic cells into iPSCs (V2.2)
are fully functioning pluripotent cells that are created by reprogramming adult somatic, or non-sex, cells, such as skin cells. By introducing four (or even fewer) genes into adult somatic cells, researchers are able to turn the differentiated adult cells back into completely undifferentiated pluripotent stem cells.
The characteristics of the induced pluripotent stem cells (iPSCs) match those of embryonic pluripotent stem cells; even mouse iPSCs completely match hESCs in shape and function. This new method of recreating stem cells from adult cells revolutionizes the way we can use cells in tissue culture. iPSCs remove the ethical stigma surrounding the use of embryonic stem cells.
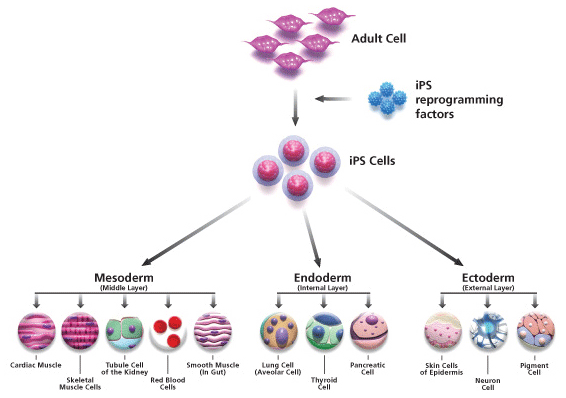
Diagram of the process of creating and then differentiating iPSCs (I2.4)
Diagram describing the process of creating the 4 types of scaffolds (I2.5)

THE FOUR TYPES OF SCAFFOLDS:
-
Pre-made porous scaffolds
-
Decellularized extracellular matrix (ECM)
-
Cell sheet
-
Hydrogel matrices
CELL SHEET WITH SECRETED ECM

The creation of engineered tissues using cell sheet with secreted ECM (I2.13)
are created with the natural secretions of closely-packed cell samples. The ECM secretions build layer upon layer of scaffold upon which the cells grow. While this method is optimal for engineering thin tissues like corneas, it's ineffective at creating thicker tissues. Intermolecular bonds and polarity (as seen in the image to the right) affect the binding of the cells to the matrix.
DECELLULARIZED EXTRACELLULAR MATRIX SCAFFOLDS
are just as the name suggests: scaffolds made of the extracellular matrix that remains after the cells have been removed. Cells are removed using a combination of enzymatic, physical, and chemical methods.
Since these scaffolds are derived from outside sources, allogeneic and xenogeneic cells must be removed so as to not trigger a negative immune response in the patient. The natural origins of this scaffold make it easy to integrate in living systems.

Image of a decellularized extracellular matrix (I2.12)
HYDROGEL MATRICES
are the products of encapsulation, which surrounds cells with a self-assembled semipermeable membrane made of hydrogels. These hydrogels are composed of crosslinked hydrated polymers, such as natural chitosan and synthetic polyethylene glycol (PEG).

An image outlining the use of hydrogels in tissue engineering scaffolding (I2.14)
PRE-MADE POROUS SCAFFOLDS

The process of making porous scaffolds (I2.11)
date back to the beginnings of scaffolding and TE, and are the most tried-and-true form of scaffolds. They are made of degradable biological substances that provide spaces for cells to grow, thus producing a structured and fully functioning organ or tissue. Pre-made porous scaffolds can be made from both natural (collagen, chitin, inorganic ceramics etc.) and synthetic sources (synthetic polymers and bioglasses).
SCAFFOLD CONSTRUCTION METHODS
Scaffold used for heart tissue repair (I2.21)
There is a multitude of ways to construct scaffolds. Scientists use methods like 3-D printing, electrospinning, and self-assembly to construct scaffolds for a tissue engineered product.
The most general method of creating a porous scaffold is by filling a mold with a polymer of choice and gelatin spheres. Pressure is then exerted on the mold at 35℃ which allows the polymers to bind together. The mold is then immersed in water which causes the gelatin to flow out of the mold. The scaffold is now perforated and ready for use.

3-D PRINTED SCAFFOLDS
3D printers can print scaffolds using both natural and synthetic biomaterials. Just by designing a blueprint, the 3-D printer can form a scaffold layer by layer, with the biomaterial of choice. 3D printers produces more scaffolds in shorter amounts of time compared to naturally-assembled scaffolds as a result of rapid and automated mechanisms.
3-D printer constructing the computer generated model of porous fibroin scaffold on the right (I2.22)

ELECTROSPINNING
Summarization of electrospinning and demonstration of mechanism (V2.3)
Electrospinning is a scaffold creation technique which uses electricity to form nanofibers from synthetic and natural materials, or both.
Electrospinning is based off the electrospray phenomenon, but instead of forming small droplets, the spinning technique forms a continuous strand of nanometer-thin fibers.
A high voltage is applied to needle in which a slowly ejected droplet of material becomes electrically charged. Electrostatic repulsion negates surface tension, ultimately stretching out the droplet. At a certain critical point, electrostatic repulsion causes the material forms a stream of liquid. The electrically charged stream is then attracted to the oppositely charged collection plate to gather the product, which is a uniform, fibrous structure.
Electrospinning is very versatile because parameters can be changed to create a plethora of distinct products. Parameters such as needle size, voltage, and the distance from collection plate can change the overall outcome of the scaffold. Electrospinning is also versatile in that it can create scaffolds made of multiple materials of different combinations. For example, scientists can test the viability of a collagen and polyurethane composite for cell growth.
Physics of electrospinning (I2.23)

SELF-ASSEMBLED PEPTIDES
Self-assembly is another method of scaffold construction which is produced from the polymerization of repeated peptide sequences. Secondary and tertiary structures which are formed formed by the peptide chain depend on the way the hydrogen bonding of the peptide back one and interactions bettwen different “R” groups that repeat throughout the chain. One example is EAK 16-II, a 16-amino acid peptide, forms stable β-sheet structures and can self assemble in hydrogels. Altering parameters like pH can also change the overall structure and formation of the scaffold, making self-assembled peptide scaffolds very versatile.
Levels of peptide molecule organization in a self-assembled scaffold (I2.24)

USING BIOREACTORS FOR TISSUE GROWTH
Bioreactors are in vitro systems which grow up tissue engineered products by regulating environmental factors (pH, humidity, temperature, gas exchange, etc.) and supplying nutrients to optimize cell proliferation. They intend to closely mimic in vivo (within the living) conditions by changing the physical, chemical, and biological parameters which can help grow tissue more similar to native ones. Bioreactors promote more cellular growth and better organize cells in a 3-D scaffold which outperforms current standard tissue culture techniques. Though tissue engineering bioreactors are still very complex and developing, they will provide an easy and safe method of tissue culturing in the future.
Muscle tissue in a simple bioreactor. Note that the bioreactor mimics muscle movements by contracting back and forth (V2.4)
That is how you make a tissue engineered product!
Click here to read more about the history of tissue engineering.

ABOUT
If the cells and the scaffold are the carefully arranged pile of logs smattered with gasoline, then the biomolecules and growth factors are the sparks that ignite the process of tissue engineering. Growth factors are polypeptides (subunits of proteins) that act as cell signals by initiating "conversation" with nearby cells. These signals vary among different biomolecules, but they effectively begin the true process of cell growth and interaction between the ECM and the cells.
Growth factors on the ECM have the ability to determine the fate of the surrounding stem cells and their path of differentiation. However, growth factors are controlled by other biomaterials that either encapsulate the growth factors to prepare for pre-programmed release or chemically bind the growth factor to the ECM.
Many different biomaterials are used to control the growth factors, such as synthetic polymers (polyamino acids, polyanhydrides, polyorthoesters, etc.).
Encapsulation, as mentioned before, surrounds cells in gels. This is done in order to preseve the efficacy of the growth factors until they're needed. By shielding the growth factors from potentially harsh conditions, the process of encapsulation allows the entire process of tissue engineering to be more effective.

A diagram detailing the function growth factors play in the cells of engineered tissues (I2.19)

Photo of encapsulated cells in biomaterials, like those used to encapsulate growth factors (I2.20)







